MODULATION OF P – GLYCOPROTEIN AT THE BLOOD BRAIN BARRIER
Author: Rimita Chakraborty
What is Blood Brain Barrier?
The Blood Brain Barrier (BBB) is a specialized structure that is composed of Endothelial cells helps to regulate the entry of compounds into the brain to maintain brain homeostasis which is essential to maintain a stable internal environment into the Central Nervous System.
P – Glycoprotein is a crucial efflux transporter which is expressed in endothelial cells of blood brain barrier (BBB). With the help of its ATP dependent mechanism, P Glycoprotein helps in the transportation of a broad range of substrates including drugs, toxins, and metabolites out of brain parenchyma and back into the bloodstream.Dysfunction of this mechanism can cause various neurological disorders including brain tumor, Alzheimer’s etc, which can lead to the changes in the effectiveness of the medication and potential neurotoxicity.
Introduction:
P – glycoprotein is also known as multi drug resistance and is mainly encoded by the MDR subtype of ABCB1 genes located in the chromosome number 7. Juliano and Ling first described P Glycoprotein in 1975. P Glycoprotein is a well identified membrane transporter with drug efflux capability. It is a membrane bound protein found in various cells of the human body. It helps in the removal of toxic materials, excess drugs, and other chemicals from the cells by using ATP hydrolysis. The majority of normal cells typically have a lower amount of expression of P Glycoprotein. P Glycoprotein is a single glycosylated polypeptide of about 170-180 KDa. It is made up of 1280 amino acids. It is found in the plasma membrane of cells. It mainly comprises the transmembrane domain divided into two homologous asymmetric halves (TMD 1 and TMD 2). Each transmembrane domain is divided into six alpha helicase domains. The TMD 1 contains 1-6 alpha helicase while the TMD 2 contains 7-12 alpha helicase. It also contains one ATP binding domain or nucleotide binding domain(NBD) comprising two ATP binding sites and one intracellular binding domain(IBD) containing an intracellular flexible polypeptide loop connecting the 12 transmembrane segment. This IBD acts as the linker protein. The K427 and K1072 are the lysine and the E552 and E 1197 are the glutamate residues that help in the catabolic action of the Human P Glycoprotein.
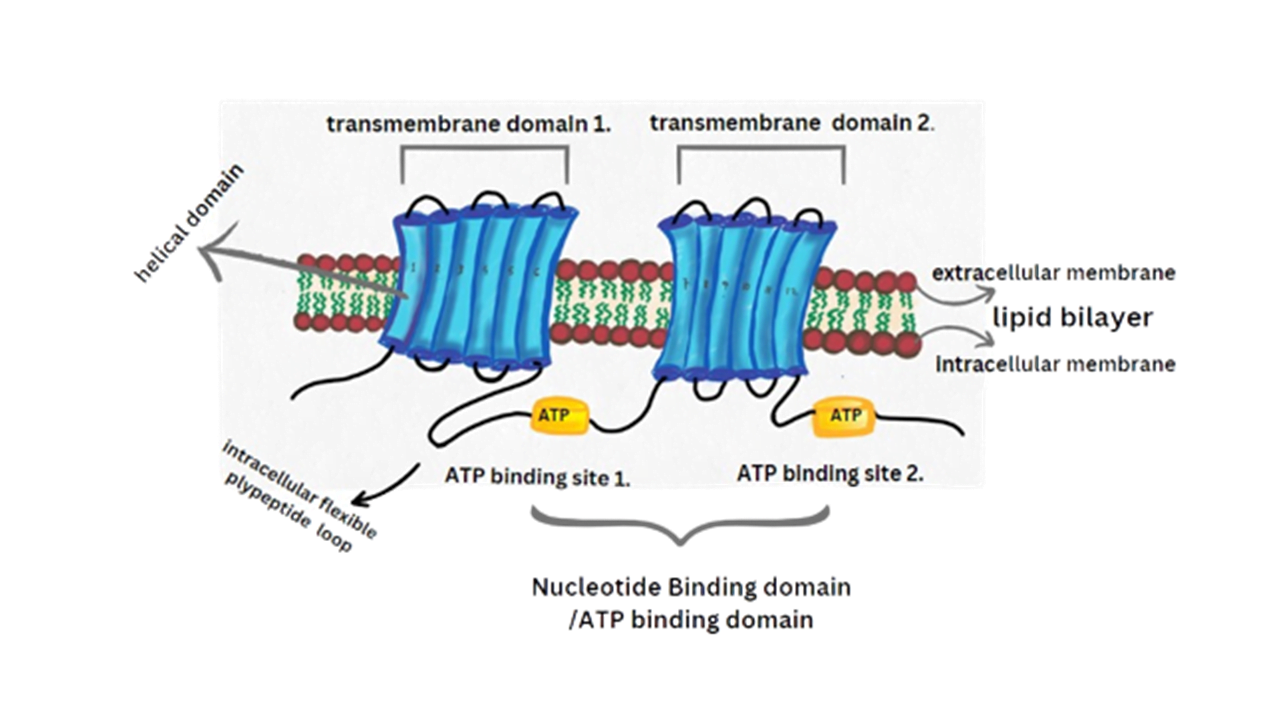 This ATP dependent drug transporter protein is located at the apical region of brain capillary endothelial cells. Presence of P Glycoprotein plays a crucial role for the protection of the central nervous system from potentially harmful substances whereas absence of P Glycoprotein in the blood brain barrier can lead to increased effect of Neurotoxicity. The function of P Glycoprotein decreases with age and this decline may be different between genders. If we closely observe the expression of P Glycoprotein across different genders then this data could contribute to age related disorders. This data will highlight the complex interplay between age, gender, transportation of drugs.
This ATP dependent drug transporter protein is located at the apical region of brain capillary endothelial cells. Presence of P Glycoprotein plays a crucial role for the protection of the central nervous system from potentially harmful substances whereas absence of P Glycoprotein in the blood brain barrier can lead to increased effect of Neurotoxicity. The function of P Glycoprotein decreases with age and this decline may be different between genders. If we closely observe the expression of P Glycoprotein across different genders then this data could contribute to age related disorders. This data will highlight the complex interplay between age, gender, transportation of drugs.
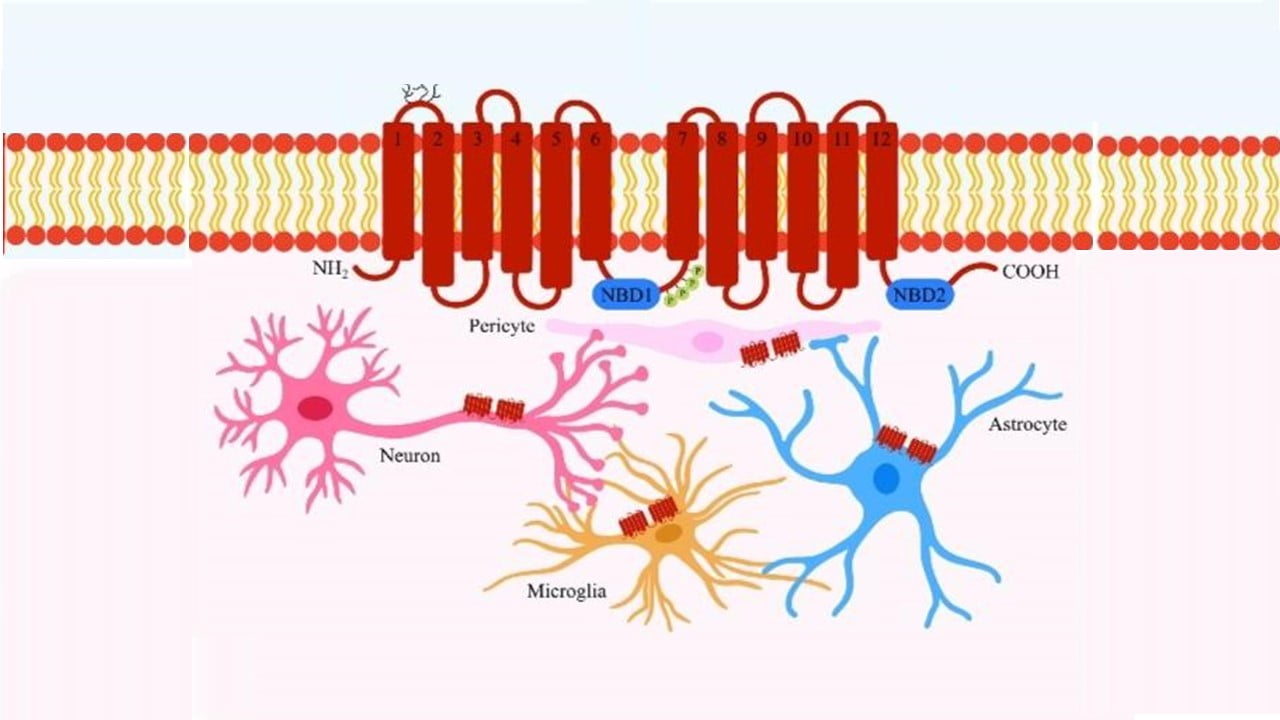
Some of the major diseases that are caused due to dysfunctioning of blood brain barrier (BBB) are Alzheimers, epilepsy, amyotrophic lateral sclerosis, multiple sclerosis. ABCB1 plays a major role in causing those diseases and influencing the mechanism behind it.
Function of Blood brain Barrier:
- It is a semipermeable membrane that helps in the regulation of passing of substances between the circulatory system and nervous system
- Helps to protect the brain from toxic substances like toxins present in the bloodstream. However certain conditions like Type 2 Diabetes increases the permeability of Blood Brain barrier that allows certain substances that are harmful for our brain which results in various neurodegenerative disorder like Alzeimer’s and Perkinson’s disease
- Helps in the transportation of essential amino acids such as Glucose, amino acids into the brain. This transportation is essential for proper neural function and signaling.
- Helps in the transportation of ions between the blood and the brain
- Transports waste products like CO2, lactate etc from the brain to the bloodstream for the purpose of Elimination
- Helps in reducing the risk of neuroinflammation and neurodegenerative diseases.
Regulation of P Glycoprotein expression:
The expression of P Glycoprotein can suppressed or enhanced in the blood brain barrier (BBB) due to several reasons.Some of them are:
The modulation of the expression of genes at the transcriptional level is known as transcriptional regulation. The MDR1 gene, which codes protein P-gp, is expressed due to several transcription factors that have been found to control this process. Nuclear receptors including the constitutive androstane receptor (CAR), glucocorticoid receptor (GR), and pregnane X receptor (PXR) are examples of these transcription factors. P-gp expression in the BBB may be altered by these transcription factors binding to particular response elements in the MDR1 gene’s promoter region. This can either increase or decrease the MDR1 gene’s transcription.
Expression of P Glycoprotein may be altered due to epigenetic modification such as DNA methylation, Histone modifications etc. which results in the changes of gene expression without altering the genetic sequences. Epigenetic modifications play an important role in the regulation of P Glycoprotein expression in the blood brain barrier. The transcriptional functioning of the MDR1 gene promoter can be influenced by DNA methylation, which is the addition of methyl groups to cytosine residues in CpG dinucleotides. This process affects the promoter’s accessibility to transcription factors. By changing DNA’s accessibility to transcriptional machinery, histone modifications such as acetylation, methylation, phosphorylation, and ubiquitination can also control chromatin shape and gene expression.
Covalent changes to proteins that occur after translation can have an impact on the stability, localization, structure, and function of the proteins. P-gp can have its activity and expression in the BBB modulated by a variety of PTMs, such as phosphorylation, glycosylation, ubiquitination, and palmitoylation. For instance, the P-gp protein’s ATPase activity and substrate transport function can be controlled by the phosphorylation of particular serine and threonine residues in the protein. P-gp trafficking and turnover at the plasma membrane have also been linked to the processes of glycosylation and ubiquitination.
Factors Influencing P Glycoprotein activity:
P Glycoprotein is a transmembrane Phosphogycoprotein which is responsible for pumping out drugs and other harmful substances from cells. However the function of P Glycoprotein can be modified due to several factors and one of the major factors is drugs.
Modulation of P Glycoprotein by drugs:
The major function of P Glycoprotein is to pump out foreign substances including drugs. Thus P Glycoprotein is essential for absorption and elimination of drugs.In this way P Glycoprotein acts as an Drug Efflux Pump.
P Glycoprotein is found in various tissues of our body including Liver, intestines and blood brain barrier. P Glycoprotein is also known as the “vacuum-cleaner model” as it uses all energy in removing the excess toxins and different by-products out of the cell. But in cancer cells the main function of P glycoprotein is to remove the drugs that are incorporated in the cancer cells to kill them. It uses ATP hydrolysis for removal of the drugs out of the cells. Some Examples include: Chemotherapeutic drugs like Doxorubicin, and immunosuppressants like cyclosporin.
There are certain drugs which are responsible for blocking the activity of P Glycoprotein. These drugs are known as inhibitors. This situation causes an increase in the intracellular concentrations of co-administered substrate medicines by inhibiting P-gp action. This may lead to increased toxicity or efficacy of the medication. P-gp can be inhibited by non-competitive or competitive methods.
Inducers are responsible for enhancing the expression and activity of P Glycoprotein. As a result of increased P-gp expression and activity, inducers improve the efflux of substrates from cells. As a result, the intracellular concentrations of concurrently delivered medications may fall, which may lessen their effectiveness. Nuclear receptors like the constitutive androstane receptor (CAR) and the pregnane X receptor (PXR) are frequently activated to induce P-gp.
| Inhibitors | Inducers | Substrates |
| Clarithromycin / Erythromycin | Carbamazepine | Colchicine |
| Amiodarone | Rifampicin | Digoxin |
| Ketoconazole / Itraconazole | St John’s Wort | Loperamide |
| Diltiazem | ||
| Quinidine | ||
| Protease inhibitors | ||
| Sirolimus / Tacrolimus | ||
| Grapefruit juice | ||
| Ciclosporin | ||
| Verapamil |
Modulation of P Glycoprotein due to various diseases:
P Glycoprotein expression is significantly elevated in the different cells affected with cancer. This leads to the upregulation of the P Glycoprotein in the cancer cells.
Breast Cancer: Expression of P Glycoprotein may vary among different subtypes of Breast cancer as mentioned above. It results in multidrug resistance in certain subtypes.
Ovarian cancer: Expression of P Glycoprotein is often observed in Ovarian cancer which results in the resistance of chemotherapeutic drugs
Colon Cancer: P Glycoprotein is also found in colon cancer which results in less effectiveness of chemotherapeutic drugs
Lung cancer: Expression of P Glycoprotein is found in non small cell lung cancer (NSLC) which affects the response to Chemotherapy
Brain tumor: P Glycoprotein expression is observed in Glioblastoma, a type of brain tumor which results in the difficulty of treatment
Prostate cancer: P Glycoprotein expression may occur in some cases of breast cancer which affect the response of certain chemotherapeutic drugs
Kidney cancer: Expression of P Glycoprotein is found in renal cell carcinoma which is a common type of kidney cancer that can impact the effect of targeted therapy.
The inflammatory bowel disease (IBD), Alzheimer’s disease, and several types of cancer have all been related to altered P-gp function. Reduced P-gp expression in the inflammatory bowel disease can enhance drug toxicity and disease vulnerability, whereas increasing P-gp expression can provide treatment resistance.
Modulation of P Glycoprotein due to Genetic Polymorphism:
P-gp is encoded by the ABCB1 gene, and variations in this gene can impact P-gp expression and activity in several tissues, including the liver, stomach, and heart. In many ethnic populations, these polymorphisms have been linked to changes in medication response and disposition, including adverse events with distinct ABCB1 substrates. But the findings have been contradictory and had little practical significance.
Clinical implications:
Because P-gp actively takes P-gp substrate medications out of the brain, decreasing their therapeutic impact, P-gp acts as a major barrier to the Central nervous system administration of P-gp substrate medications. P-gp activity modification can enhance the effectiveness of CNS drugs by improving drug transport to the brain.
Conclusion:
Modulation of P Glycoprotein in the blood brain barrier can help deliver the appropriate drugs in the brain and can help to treat Neurological disorders. It can offer new insights in the field of neurotherapeutics.
References:
- Schinkel, A. H. (1999, April). P-Glycoprotein, a gatekeeper in the blood–brain barrier. Advanced Drug Delivery Reviews, 36(2–3), 179–194. https://doi.org/10.1016/s0169-409x(98)00085-4
- van Assema, D. M. E., Lubberink, M., Boellaard, R., Schuit, R. C., Windhorst, A. D., Scheltens, P., Lammertsma, A. A., & van Berckel, B. N. M. (2012, April 3). P-Glycoprotein Function at the Blood–Brain Barrier: Effects of Age and Gender. Molecular Imaging and Biology, 14(6), 771–776. https://doi.org/10.1007/s11307-012-0556-0
- Bauer, M., Tournier, N., & Langer, O. (2019, March 23). Imaging P‐Glycoprotein Function at the Blood–Brain Barrier as a Determinant of the Variability in Response to Central Nervous System Drugs. Clinical Pharmacology & Therapeutics, 105(5), 1061–1064. https://doi.org/10.1002/cpt.1402
- Chai, A. B., Callaghan, R., & Gelissen, I. C. (2022, November 24). Regulation of P-Glycoprotein in the Brain. International Journal of Molecular Sciences, 23(23), 14667. https://doi.org/10.3390/ijms232314667
- Mossel, P., Bartels, A. L., de Deyn, P. P., & Luurtsema, G. (2020, December 15). The Role of P-Glycoprotein at the Blood–Brain Barrier in Neurological and Psychiatric Disease. PET And SPECT in Psychiatry, 45–81. https://doi.org/10.1007/978-3-030-57231-0_3
- Attia, M. S., Elsebaey, M. T., Yahya, G., Chopra, H., Marzouk, M. A., Yahya, A., & Abdelkhalek, A. S. (2023, March). Pharmaceutical polymers and P-glycoprotein: Current trends and possible outcomes in drug delivery. Materials Today Communications, 34, 105318. https://doi.org/10.1016/j.mtcomm.2023.105318
- Daneman, R., & Prat, A. (2015, January). The Blood–Brain Barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. https://doi.org/10.1101/cshperspect.a020412
- Kadry, H., Noorani, B., & Cucullo, L. (2020, November 18). A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS, 17(1). https://doi.org/10.1186/s12987-020-00230-3
- Cho, H., Lee, H. Y., Han, M., Choi, J. R., Ahn, S., Lee, T., Chang, Y., & Park, J. (2016, August 11). Localized Down-regulation of P-glycoprotein by Focused Ultrasound and Microbubbles induced Blood-Brain Barrier Disruption in Rat Brain. Scientific Reports, 6(1). https://doi.org/10.1038/srep31201
- Iqbal, M., Gibb, W., & Matthews, S. G. (2011, March 1). Corticosteroid Regulation of P-Glycoprotein in the Developing Blood-Brain Barrier. Endocrinology, 152(3), 1067–1079. https://doi.org/10.1210/en.2010-1227
- Ding, Y., Wang, R., Zhang, J., Zhao, A., Lu, H., Li, W., Wang, C., & Yuan, X. (2019, August 5). Potential Regulation Mechanisms of P-gp in the Blood-Brain Barrier in Hypoxia. Current Pharmaceutical Design, 25(10), 1041–1051. https://doi.org/10.2174/1381612825666190610140153
- Abdullahi, W., Davis, T. P., & Ronaldson, P. T. (2017, April 26). Functional Expression of P-glycoprotein and Organic Anion Transporting Polypeptides at the Blood-Brain Barrier: Understanding Transport Mechanisms for Improved CNS Drug Delivery? The AAPS Journal, 19(4), 931–939. https://doi.org/10.1208/s12248-017-0081-9
- Tanigawara, Y. (2000, February). Role of P-Glycoprotein in Drug Disposition. Therapeutic Drug Monitoring, 22(1), 137–140. https://doi.org/10.1097/00007691-200002000-00029
- Rodriguez, I., Abernethy, D. R., & Woosley, R. L. (1999, February 2). P-Glycoprotein in Clinical Cardiology. Circulation, 99(4), 472–474. https://doi.org/10.1161/01.cir.99.4.472
- Lin, J. H., & Yamazaki, M. (2003). Role of P-Glycoprotein in Pharmacokinetics. Clinical Pharmacokinetics, 42(1), 59–98. https://doi.org/10.2165/00003088-200342010-00003
- Medicines interactions: the role of P-glycoprotein. (n.d.). https://www.medsafe.govt.nz/profs/puarticles/p-glycoproteinsept2011.htm
- P-glycoprotein. (2024, April 29). Wikipedia. https://en.wikipedia.org/wiki/P-glycoprotein
- Chai, A. B., Callaghan, R., & Gelissen, I. C. (2022, November 24). Regulation of P-Glycoprotein in the Brain. International Journal of Molecular Sciences, 23(23), 14667. https://doi.org/10.3390/ijms232314667
- Ahmed Juvale, I. I., Abdul Hamid, A. A., Abd Halim, K. B., & Che Has, A. T. (2022, June). P-glycoprotein: new insights into structure, physiological function, regulation and alterations in disease. Heliyon, 8(6), e09777. https://doi.org/10.1016/j.heliyon.2022.e09777
- Liu, L., Collier, A. C., Link, J. M., Domino, K. B., Mankoff, D. A., Eary, J. F., Spiekerman, C. F., Hsiao, P., Deo, A. K., & Unadkat, J. D. (2015, September 9). Modulation of P-glycoprotein at the Human Blood-Brain Barrier by Quinidine or Rifampin Treatment: A Positron Emission Tomography Imaging Study. Drug Metabolism and Disposition, 43(11), 1795–1804. https://doi.org/10.1124/dmd.114.058685
- Han, L. (2021, November 27). Modulation of the Blood–Brain Barrier for Drug Delivery to Brain. Pharmaceutics, 13(12), 2024. https://doi.org/10.3390/pharmaceutics13122024
