Fading Away: The Science Behind Cellular Death
Author: Atharva Tilewale
Abstract
Cell death is a critical biological phenomenon wherein a cell ceases to function. This process, essential for maintaining organismal homeostasis, occurs through various mechanisms, including programmed cell death and pathological factors like diseases or injuries. Understanding the reasons behind cell demise is pivotal, as it serves multiple purposes: eliminating redundant cells during development, forming structural components like the skin, clearing excess cells post-infection, removing damaged cells from exposure to radiation or toxins, and combatting viral infections.
Introduction
Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, as in programmed cell death, or may result from factors such as diseases, localised injury, or the death of the organism of which the cells are part.
Why do cells die?
Cell death is an important process in the body. It removes cells in situations including:
- When cells are not needed, such as during certain stages of development.
- To create a structure in the body, for example, the outer layer of the skin is made of dead cells.
- To remove excess cells, such as white blood cells after an infection has been cleared.
- If cells are damaged, such as by radiation or toxins.
- When cells are infected by viruses.
Cell death proteins
Many proteins have been discovered that control whether a cell dies by the processes of apoptosis, necroptosis or pyroptosis. Some key cell death control proteins include:
- Caspases: these enzymes are switched on in apoptotic cells, and digest other proteins to bring about cell death. Some caspases have roles in processes other than cell death.
- Bcl-2 family proteins: these proteins interact with each other to determine whether a cell undergoes apoptosis or stays alive. Some Bcl-2 family proteins promote survival and block apoptosis. Others are ‘pro-death’, and trigger apoptosis.
- Death receptors: these are proteins on the surface of the cell. When they are bound by certain cytokines (hormone-like signalling proteins), they cause changes in the cell that can lead to cell death.
- RIP kinases: two proteins called ‘RIP1 kinase’ and ‘RIP3 kinase’ trigger necroptosis.
- IAPs: or ‘inhibitors of apoptosis proteins’ can prevent cell death. They can do this by blocking several cell death proteins including caspases and RIP1 kinase.
- SMAC/Diablo: is an inhibitor of IAPs. In healthy cells, SMAC is stored away from IAPs, in parts of the cell called mitochondria. When cell death is triggered, SMAC can leak out and block the IAPs function. Thus, the release of SMAC out of mitochondria can promote cell death.
Apoptosis
Apoptosis is the programmed cell death that occurs in multicellular organisms, which proceeds by a distinct series of cellular changes. It is caspase-mediated cell death. It has the following morphological features:
- blebbing
- cell shrinkage
- cytoplasmic and chromatin condensation
- nuclear fragmentation
- formation of apoptotic bodies
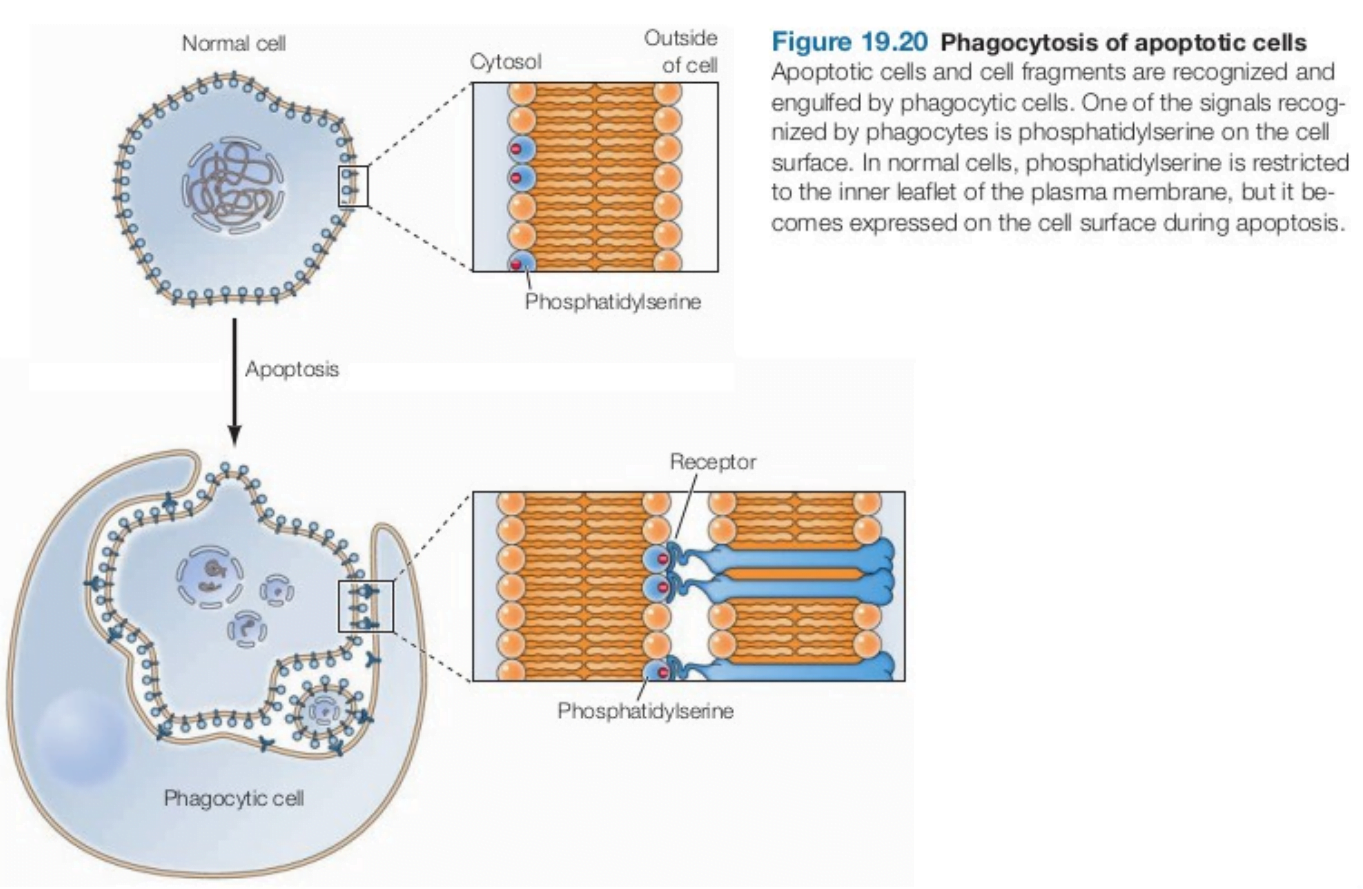
Apoptotic cells and cell fragments are phagocytosed. It is mediated by an “eat me” signal (i.e. phosphatidylserine) on the cell surface. It is normally present on the inner leaflet of plasma membrane but during apoptosis, phosphatidylserine becomes expressed on the cell surface, where it is recognised by receptors expressed by phagocytic cells. Apoptotic pathways are of 2 types: Intrinsic and Extrinsic. The intrinsic pathway is activated by DNA damage and other forms of cell stress, whereas the extrinsic pathway is activated by signals from other cells.
Caspases
Caspases are the protease which are effectors or executioners of programmed cell death, bringing about the events of apoptosis by cleaving more than 100 different target proteins. One of its targets is an inhibitor of a DNase, which when activated is responsible for fragmentation of nuclear DNA. It cleaves
- nuclear lamins, leading to fragmentation of the nucleus;
- cytoskeletal proteins, leading to disruption of the cytoskeleton, blebbing (irregular bulging) of the plasma membrane, and cell fragmentation; and
- Golgi matrix proteins, leading to fragmentation of the Golgi apparatus.
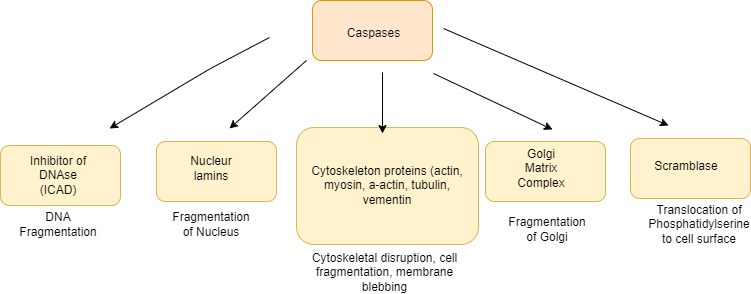
- Caspases also cleave and activate a “scramblase” that translocates phosphatidylserine from the inner to the outer leaflet of the plasma membrane.
- All caspases are synthesized as inactive precursors (called procaspases) that can be converted to the active form by proteolytic cleavage, catalyzed by other caspases.
- Initiator caspases are activated directly in response to the various signals that induce apoptosis. The initiator caspases then cleave and activate the effector caspases. The activation of an initiator caspase therefore starts off a chain reaction of caspase activation leading to death of the cell.
Caspases have been broadly classified by their known roles in apoptosis (caspase-3, -6, -7, -8, and -9 in mammals), and in inflammation (caspase-1, -4, -5, -12 in humans and caspase-1, -11, and -12 in mice). Caspases involved in apoptosis have been subclassified by their mechanism of action and are either initiator caspases (caspase-8 and -9) or executioner caspases (caspase-3, -6, and -7).
Apoptosis: Intrinsic Pathway (Mitochondrial pathway)
- DNA damage and other forms of cell stress induces the intrinsic pathway of apoptosis.
- DNA damage activates two protein: ATM and chk2 protein kinase.
- This protein phosphorylate and stabilize p53.
- p53 activates Bax and Bak by the expression of proapoptotic regulatory protein.
- Bax and Bak oligomerize to form pores in the mitochondrial outer membrane, leading to the release of cytochrome c from the mitochondrial intermembrane space.
- The release of cytochrome c then triggers caspase activation (in mammals caspase-9).
- It is activated by forming a complex with Apaf-1 in a multisubunit complex called the apoptosome (caspase-9 + cytochrome c + Apaf-1).
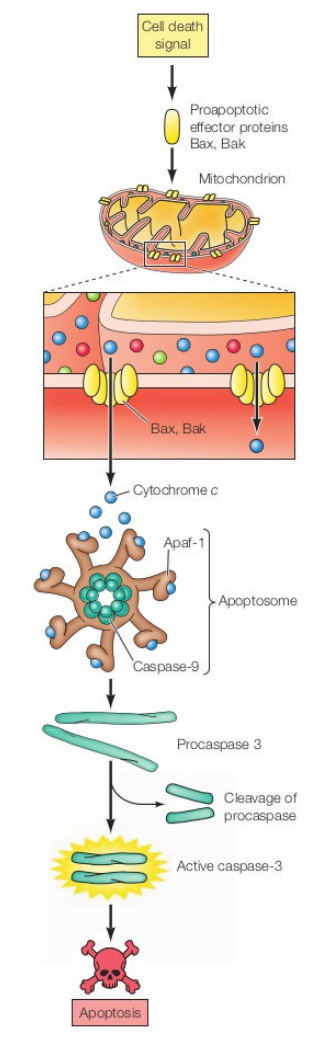
- Under normal conditions of cell survival, cytochrome c is localized to the mitochondrial intermembrane space while Apaf-1 and caspase-9 are found in the cytosol, so caspase-9 remains inactive.
- Once activated, caspase-9 cleaves and activates downstream effector caspases, such as caspase-3
- Caspase-3 degrades the inhibitor of nuclease and protease. Now nuclease becomes active and degrades the nucleus, eventually resulting in cell death.
Apoptosis: Extrinsic Pathway
- Extrinsic pathway is activated by signals from other cells.
- The caspases are activated by death receptors.
- One death ligand is the Tumor Necrosis Factor (TNF)-Related Apoptosis-Inducing Ligand (TRAIL), and a member of the TNF family of ligands (Fas, TNF-alpha, etc.)
- The TRAIL receptors, induce apoptosis by recruiting, via death domain (DD) interaction, adaptor proteins such as Fas-Associated protein with Death Domain (FADD) and apical procaspases such as procaspase-8. It results in the assembly that forms the Death Inducing Signaling Complex (DISC).
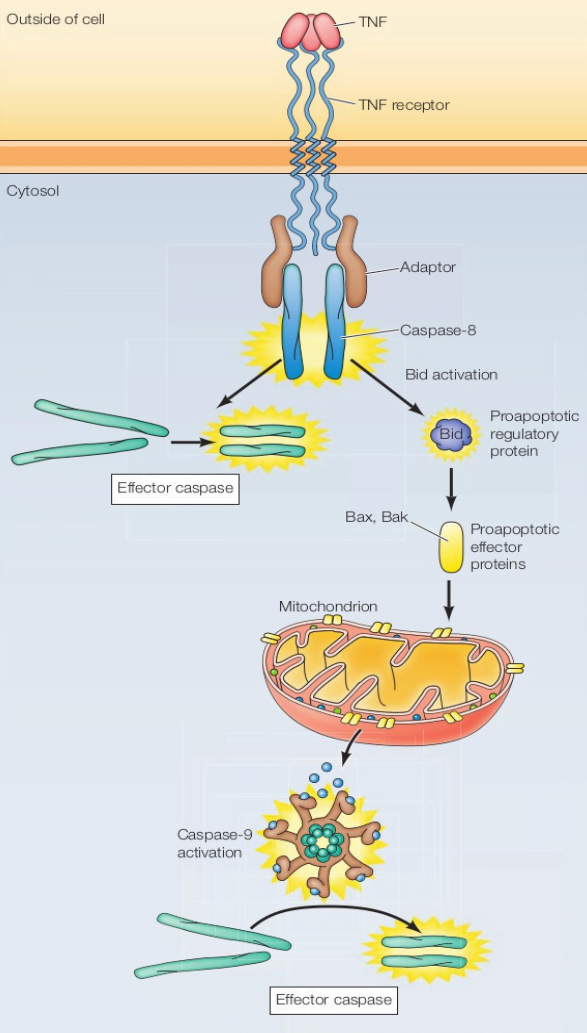
- It promotes the auto-proteolytic activation and release of apical caspases.
- In most tumor cells, the activated apical caspases then initiate two main pathways of cell death.
- In one pathway (the extrinsic), caspase-8 activation directly activates effector caspase-3, which facilitates apoptosis.
- Alternatively, the death receptor pathway can also activate the mitochondrial pathway (intrinsic pathway).
- In the mitochondrial pathway, changes in the mitochondrial membranes lead to the release of apoptogenic factors such as cytochrome c.
- Upon entry into the cytosol, cytochrome c binds the caspase-activating protein Apaf1, stimulating the binding of Apaf1 to procaspase-9, forming apoptosome and subsequently inducing processing and activation of caspase-9.
- As a result, activated caspase-9 is then available to cooperate with caspase-8 in the processing and activation of caspase-3, leading to the demise of the cell.
Necrosis
Necrosis is a form of cell injury which results in the premature death of cells in living tissue by autolysis. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated digestion of cell components. Cellular death due to necrosis result in the loss of cell membrane integrity and an uncontrolled release of products of cell death into the extracellular space. This initiates in the surrounding tissue an inflammatory response, which attracts leukocytes and nearby phagocytes which eliminate the dead cells by phagocytosis.
Autophagy
Autophagy, a kind of intracellular degradation process, is used to remove proteins and/or ageing, damaged organelles due to folding errors. Autophagy refers to the formation of autophagosomes by membrane wrapping part of the cytoplasm and the organelles and proteins that need to be degraded in the cells. Autophagosomes are fused with lysosomes to form autophagolysosome, which degrade the contents of the inclusions, to achieve cell homeostasis and organelle renewal. Autophagy is a phenomenon of “self-eating” in cells. Autophagy is divided into three processes: autophagy initiation, autophagy membrane extension and autophagy lysosome formation.
When the autophagosome membrane appears, autophagy is generally divided into three categories according to the different pathways of cell material to lysosomes, such as:
- macroautophagy, which is formed by endoplasmic reticulum-derived membrane wrapping around the degradation material and then melted with a lysosome to degrade its contents;
- small autophagy (microautophagy), direct phagocytosis by lysosome to degrade long-lived proteins in cells;
- Chaperone-mediated autophagy, (CMA), the process by which intracellular proteins (soluble) bind to molecular chaperones and are transported to lysosomes and then digested by lysosomal enzymes.
Moreover, according to nutritional status, autophagy is divided into non-selective autophagy under starvation conditions, and selective autophagy under nutrient-rich conditions, such as mitochondrial autophagy (Mitophagy), aggregate autophagy (Aggrephagy), peroxisome autophagy, etc.
Necroptosis
Necroptosis is a caspase-independent death program. Necroptosis is a regulated form of necrotic cell death that critically depends on receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like (MLKL). Apoptosis and necroptosis are closely related, and TNF can determine the final fate of cells.
Mechanism
- TNF binds to TNF receptor 1.
- Receptor recruits the death domain (DD)-containing adaptor proteins TRADD, TRAF2, and RIP1 to form the so-called complex I.
- Several components of complex I recombine to form a cytosolic complex (complex II) that recruits FADD (Fas-associated via DD) via DD-mediated interactions.
- In complex II, FADD recruits procaspase-8, whilst RIP1 recruits RIP3.
- When RIP3 is high, complex II tends to recruit large amounts of this protein and turns itself into a so-called necrosome.
- RIP3 is autophosphorylated.
- Auto-phosphorylated RIP3 recruits and phosphorylates the mixed-lineage kinase domain-like protein (MLKL).
- This leads to MLKL oligomerization and translocation to the plasma membrane. MLKL oligomers execute necroptosis by generating cation channels causing plasma membrane rupture.
When RIP3 is low, caspase-8 can activate and it will undergo apoptosis. In necrosome, procaspase-8 cleaves RIP1 and RIP3 which prevents the initiation of necroptosis. So for necroptosis to occur, caspase-8 activity must be inhibited.
Conclusion
In conclusion, cell death is a fundamental aspect of biological processes, playing crucial roles in development, tissue homeostasis, and disease pathology. Whether through programmed mechanisms like apoptosis or in response to external insults, the cessation of cell function serves various purposes, from sculpting tissues during embryogenesis to removing damaged or infected cells to preserve organismal integrity. Understanding the intricacies of cell death is essential for unraveling disease mechanisms and developing therapeutic interventions. Moving forward, continued research into the regulation and consequences of cell death will deepen our understanding of cellular biology and its implications for human health and disease.
References
- The Cell – A Molecular Approach by Geoffrey M Cooper, 8th edition
- Basic neurochemistry, 8th edition
- https://doi.org/10.1016/j.bulcan.2020.11.004
- https://doi.org/10.3389/fcell.2021.789948
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2941895/