
Author: Sujal Pawar
What are Monoclonal antibodies (mAbs)?
A monoclonal antibody is a homogeneous collection of monospecific antibodies. These antibodies are selective and have affinities for a single epitope of a particular antigen. Since the discovery of monovalent antibodies, their therapeutic and diagnostic applications have expanded to include a variety of biotechnology sectors such as toxicology, molecular biology, biochemistry, and medicine. Hybridoma technology is one of the most significant and commonly employed of the numerous ways (single lymph cell amplification or culture procedures) that have been developed over the years to manufacture monoclonal antibodies.
What are polyclonal antibodies (pAbs)?
Polyclonal antibodies are immunoglobulins produced by several B cell lineages. These pAbs recognize many epitopes of a single antigen. Polyclonal antibodies, which are heterogeneous mixtures of various antibodies, can recognize a wide range of epitopes. Polyclonal antibody synthesis increases the immune system’s efficacy, allowing it to respond rapidly and effectively to any bacterial or viral infection. Polyclonal sera are a valuable chemical agent with numerous applications because of their capacity to bind to multiple epitopes. Polyclonal sera have long been used to treat pathogenic and toxin-producing microorganisms.The manufacturing process introduces batch-to-batch variability, increasing the risk of transmitting bloodborne diseases.
History of Monoclonal Antibodies (mAbs)
An account of the development of monoclonal antibodies (mAbs).Kohler and Milstein developed hybridoma technique, which resulted in the discovery of monoclonal antibodies in 1975. Current study involves a breakthrough scientific process in which immortal malignant cell types, including myeloma cells, are fused with B cells that make antibodies to form a hybrid.In 1988, Gregory Winter and colleagues found the ability to manufacture humanized monoclonal antibodies. They also eliminated the side effects that some persons observed when using too many monoclonal antibodies.
Tasuku Honjo and James P. Allison were awarded the 2018 Nobel Prize in Physiology or Medicine for their research on cancer treatments that reduce negative immune control by disrupting inhibitory pathways.
What are myloma cells and why they are used?
- Myeloma (MM) is a cancer that attacks plasma cells, which are specialized white blood cells that produce antibodies.Also known as plasma cell myeloma, or just myeloma.
- Many people with multiple myeloma experience no adverse responses at first.Regardless, as the condition worsens, infections, iron insufficiency, renal failure, and bone abnormalities may develop.
- Hypercalcemia and amyloidosis are possible consequences. The exact cause of many myeloma instances is unknown.Several risk variables were discovered in each case, including weight, radiation exposure, age, family history, and chemical exposure.
Certain jobs, in particular, provide a higher risk due to the mechanical handling of odorous hydrocarbon solvents.Furthermore, cryptic central monoclonal gammopathy (MGUS) can progress to smoldering myeloma and, ultimately, full-blown other myeloma.
Hybridoma Technology
- The method of making hybridomas, which involves finding antigen-specific plasma/plasmablast cells that make antibodies particular to an antigen of intrigued and combining these cells with myeloma cells, is generally mindful for the labor included in creating monoclonal antibodies.
- A rabbit hybridoma can be made utilizing rabbit B-cells. Adjoining plasma films can be combined utilizing polyethylene glycol, but this strategy contains a limited victory rate. As a result, only combined cells can multiply within the particular medium.
- Typically conceivable since hypoxanthine-guanine-phosphoribosyl transferase (HGPRT), an protein required for the rescue synthesis of nucleic acids, can not be synthesized by myeloma cells. These cells don’t endure from the need of HGPRT unless there’s moreover a disturbance within the de novo purine union pathway.
- Below flow chat showing the steps for hybridoma technology
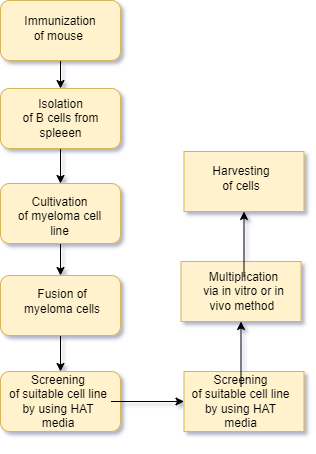
- Vaccination or Immunization
- The initial step is giving laboratory animals, such as mice or rabbits, an injection of a particular antigen against.
- It accelerates B cell differentiation into memory and plasma B cells by raising the antibodies through a series of injections given over several weeks. After a few weeks of vaccination, when a enough amount of antibodies form in the animal serum, the animal is sacrificed.
- Isolation of B lymphocytes
- In order to separate the activated B-cells, the spleen is removed under aseptic circumstances after sacrifce. Centrifugation with a density gradient is used for this process. Techniques like ELISA and flow cytometry are used to determine whether there are any antibodies present in the serum. The serum has B cells that have been activated. (that generate antibodies).
- The myeloma cells are subsequently combined with the activated B lymphocytes.
- Myloma cell line preparation
- A few weeks prior to cell fusion, metastatic tumor cells are grown in 8-azaguanine to produce non-functional hypoxanthine-guanine phosphoribosyltransferase.
- Myeloma cell genes (HGPRT). Non-functional HGPRT can lead to metastatic tumor cells becoming sensitive to HAT medium and preventing nucleotides from the salvage route from assembling when hybridoma technology is employed.
- Fusion of cells
- The process of fusing HAT-sensitive myeloma cells with activated B lymphocytes is known as cell fusion. This process involves centrifuging newly acquired HAT-sensitive myeloma cells in fusion-promoting medium along with activated B-cells. In this process, polyethylene glycol (PEG) is employed.
- PEG facilitates the fusing of cells by encouraging the myeloma cells’ plasma membrane to fuse with the antibody-producing cells’ plasma membrane. This results in the formation of a heterokaryon, a multinucleated cell. Electrofusion is an additional fusion technique that involves the fusion of cells with the application of an electric field. This approach is more effective than the one that came before it.
- Hybridoma selection: –
- In PEG-containing media, the cells merge to generate hybridoma cells.
- Even with the most effective fusion procedures, just 1–2% of hybridoma cells merge.
- Furthermore, there is around one viable hybrid cell per hundred cells. The medium has a high amount of unfused cells.
- During this stage, fused cells differentiate from unfused cells. This is done by cultivating the cell combination in HAT medium (selective media) for 10-14 days following incubation. HAT medium contains hypoxanthine, aminopterin, and thymidine. Aminopterin in HAT media hinders the cell’s capacity to generate nucleotides via the de novo pathway. The HGPRT gene is activated in the cell.
- screening of hybridoma cells
- HAT-selected hybridoma cells are transferred to ELISA plates, with each well containing a single cell.
- This is accomplished using the limited dilution method. Hybridoma cells have B cell genes that create a specific antibody with a specific epitope, known as a monoclonal antibody.
- Other hybridomas in other wells may produce antibodies that target a different epitope for the same antigen. After separating and isolating different hybridomas, screening is done to choose those that produce antibodies targeting specific epitopes for an antigen. Capable of surviving by producing hypoxanthine and deoxythymidine through salvage mechanisms. Unfused B cells have a short lifespan, dying within a few days.
- Unfused malignant neoplastic cells die because they lack the hypoxanthine-guanine phosphoribosyltransferase (HGPRT) gene. The presence of aminopterin inhibits their ability to synthesize nucleotides using the de novo method. Because of the functioning HGPRT gene from B lymphocytes, which makes them HGPRT positive, hybrid cells that are still viable in the media can grow and divide on HAT media. As a result, they can accumulate endlessly on HAT media.
- Cloning and multiplication of hybridoma cells.
Hybridomas with suitable antibodies are identified and transplanted to large culture containers. Hybridoma cell lines are cultivated either in vivo or in vitro. Hybridoma cells can be kept in culture conditions to produce monoclonal antibodies.
| In vivo method | In vitro method |
| · The in vivo approach produces monoclonal antibodies from mice. Mice are given intraperitoneally with 105-110 viable hybridoma cells. After a few weeks, the ascites fluid is collected from an anesthetized mouse. Ascites fluids are polluted with · Monoclonal antibodies can be isolated from mouse immunoglobulins but require purification. This approach may not be ideal for maintaining antibody purity
| · This method involves lab-based cultivation of hybridoma cells. The process includes cultivating hybrid cells in culture conditions and then isolating monoclonal antibodies from them. · This approach is ideal for growing hybrid cells due to its lower chance of contamination. In vitro antibody production leads.
|
Clinical application of monoclonal antibodies
- Diagnostic testing.
- Testing of pregnancy.
- Radioimmunodetection (RID) of cancer.
- Malaria herpes virus testing. Identifcation of diferent strains of pathogens.
- Serological identifcation of ABO blood groups.
- Radioimmunotherapy (RIT) of cancer.
- Cancer treatments through drug. Viral disease treatment.
- Specifc cell identifcation and their functions.
- Organ transplantation.
- Immunopurifcation
Conclusion
There is currently a risk of disease transmission from mice to people since the monoclonal antibodies used are either produced in mice or rats. Regardless of the purification technique, there is no guarantee that antibodies produced this way are virus-free. There have also been reports of immunogenic reactions to mouse-derived antibodies. Genetic engineering and other cutting-edge technical technologies helped to alleviate some of these limits. Cutting-edge procedures are being developed to produce monoclonal antibodies in the lab that are as near to human beings as possible. This overview discusses the merits and downsides of monoclonal antibody production, as well as the technique’s evolution, therapeutic significance, and future posibilities.
References
- Kuby Immunology-4th edition
- Hybridoma technology; advancements, clinical significance, and future aspects https://link.springer.com/article/10.1186/s43141-021-00264-6
- Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7255167/
